gravimetric determination of chloride as silver chloride by precipitation method|chloride anion gravimeter : private label In most cases the precipitate is the product of a simple metathesis reaction between the analyte and the precipitant; however, any reaction that generates a precipitate . Resultado da 14 de jun. de 2020 · Users share their experiences and tips on how to crossplay between Steam and Epic Games versions of Satisfactory, a PC .
{plog:ftitle_list}
Ju coura (@julianecoura) no TikTok |30M curtidas.2.2M seguidores.Sigam ️Juliane.coura ⭐️ CEO @fitstorecascavel 📱 ️ 45 99983-7935.Assista ao último vídeo de Ju coura (@julianecoura).
best moisture meter for carpet
silver ion precipitation gravimetry
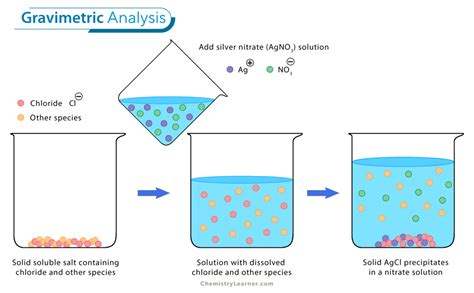
The chloride content of a soluble salt, or of an aqueous solution, can be determined by precipitation of the chloride ion as silver chloride: Ag+(aq) + Cl-(aq) → AgCl(s) loid, which is coagulated with heat. Nitric acid and a small excess of silver nitrate aid coagulation by . In most cases the precipitate is the product of a simple metathesis reaction between the analyte and the precipitant; however, any reaction that generates a precipitate .The chloride content of a soluble salt can be determined by precipitation of the chloride anion as silver chloride according to the reaction: Ag+(aq) + Cl–(aq) AgCl(s) . 1.1 . If fully precipitated, .
silver chloride concentration
Gravimetric analysis is a quantitative determination of the amount of analyte through a precipitation process, precipitate isolation, and determination of isolated product weight.
The best way to appreciate the theoretical and practical details discussed in this section is to carefully examine a typical precipitation gravimetric method. Although each method is unique, the determination of Mg 2 + in water and wastewater . For example, in the precipitate of silver chloride, one silver ion is surrounded by six chloride ions within the lattice. However, at the surface of the lattice, silver ions are not .
From the mass of AgCl it is possible to calculate the amount of chloride in your sample and finally the mass percent chloride in your unknown. This is an example of what is known as .In order to do this we will employ a Gravimetric Analysis in which the Chloride is precipitated as AgCl. This precipitate will be collected and weighed, giving us, indirectly, the amount of .
In this experiment you will conduct a gravimetric analysis of the chloride ion (Cl-) mass percentage in an unknown solid. The method makes use of the very low solubility of silver .Ask the Chatbot a Question Ask the Chatbot a Question gravimetric analysis, a method of quantitative chemical analysis in which the constituent sought is converted into a substance (of known composition) that can be separated from the sample and weighed. The steps commonly followed in gravimetric analysis are (1) preparation of a solution containing a known weight of . Precipitation Reaction; Silver Chloride; Sulfamic Acid; Gravimetric Determination; These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.silver nitrate to produce the precipitate, silver carbonate. This competitive . to permit its use without lessening the accuracy of the determination. 5.2 Reagent water. All references to water in this method refer to . Method 9253: Chloride (Titrimetric, Silver Nitrate), part of Test Methods for Evaluating Solid Waste, Physical/Chemical .
Gravimetric verification of chloride concentration from a precipitate of silver nitrate. Introduction: Gravimetric analysis is a method of quantitative chemical analysis used to determine the concentration of a constituent by converting it into a substance of known composition and separating it from the sample.Gravimetric analysis can be used to determine the concentration of an unknown chloride solution or the percentage by mass of an unknown chloride salt. A common method is to add an excess of acidified silver nitrate to a solution of the unknown salt to form a silver chloride .Silver nitrate solution causes staining of skin and fabric (chemical burns). Any spills should be rinsed with water immediately. Introduction This method determines the chloride ion concentration of a solution by titration with silver nitrate. As the silver nitrate solution is slowly added, a precipitate of silver chloride forms. Ag+ (aq) + Cl .This method determines the chloride ion concentration of a solution by gravimetric analysis. A precipitate of silver chloride is formed by adding a solution of silver (PDF) Determination of chloride Ion Concentration by Gravimetric | Farhang Awlqadr - Academia.edu
Gravimetric analysis is a quantitative method used in chemistry to determine the amount of a substance by measuring its mass. Learn types, procedure, differences etc. . Step 1: Calculate the mass of chloride in the silver chloride precipitate. \(0.7796 g AgCl x (0.1 g Cl^-/0.2478 g AgCl) = 0.314 g Cl^-\) Then slightly extra silver ions react with the chromate ions and form a brownish-red precipitate of silver chromate. The solubility product of silver chromate exceeded in the presence of additional silver ions, and then the precipitation occurs. . Mohr method is one of the significant methods for determination of chloride in water. It is also .The effective charge on two silver chloride particles In Fig.12(a), The upper curve represents a particle in a solution that contains a reasonably large excess of silver nitrate, and the lower curve depicts a particle in a solution that has a much lower silver nitrate content. Note that the .Gravimetric Analysis of Chloride In this experiment you will conduct a gravimetric analysis of the chloride ion (Cl-) mass percentage in an unknown solid. The method makes use of the very low solubility of silver halides. Gravimetry has the distinct advantage of being an extremely straightforward and direct method.
Mohr Method, 2018. Objective: Determination of chloride in solid and liquid samples by the Mohr Method Learning Outcome: • Students understand the terms volumetric analysis, morarity, molality normality and redox titration. • Students acquire the skill to prepare standard solutions of silver nitrate and sodium chloride.
The precipitate must be a pure compound. The precipitate must be easily filtered. An example of a gravimetric analysis is the determination of chloride in a compound. In order to do a gravimetric analysis, a cation must be found that forms an insoluble compound with chloride. This compound must also be pure and easily filtered.allow for its direct precipitation. In the gravimetric analysis of chloride experiment, the AgCl precipitate must be quantitatively recovered and then washed to remove excess Ag+. Washing the precipitate with pure water can result in peptization of the precipitate - the breakup of the coagulated colloid into colloidal particles.
Identifying an unknown chloride salt by gravimetric analysis Summary Gravimetric analysis will be performed to identify an unknown chloride salt. This method of analysis allows for a quantitative determination of the mass percent of chlorine in the unknown through precipitation of the chloride ions in the form of silver chloride.wanted. This is our first exposure in this class to a Gravimetric analysis – one of the oldest and best-established analytical methods we consider this term. Precipitation Gravimetry Gravimetric analysis is a standard classical method for determining the amount of a given component present in a host of solid and solution sample types. Precipitation gravimetric analysis separates ions from a solution by using the precipitation process (the reaction that creates an insoluble solid product from the reaction of two soluble solid products). . the white .Gravimetric Determination of Silver Chloride in virtual laboratory analysis bio 213_analytical methods for biology gravimetric determination of silver chloride. Skip to document. University; High School . In the 17th century, the alchemists and Robert Boyle used common salt to precipitate silver from cyanide solutions as silver chloride (AgCl
1 Gravimetric*Determination*of*Chloride* * Introduction* * Thechloridecontentofasolublesalt,orofanaqueoussolution,canbe determined*by*precipitation*of*the*chloride .The two most common methods for the determination of chloride ion are based on its quantitative reaction with silver ion to form silver chloride. One method involves the isolation of the silver chloride precipitate by filtration, determining its mass, and using stoichiometry to calculate the percent chloride in the sample, this is termed .
precipitation gravimeter solution
chloride, bromide and cyanide ions by reacting with silver ions to form a brick-red silver chromate precipitate in the equivalence point region. The Mohr method uses chromate ions as an indicator in the titration of chloride ions with a silver nitrate standard solution. After all the chloride has been precipitated as white silver chloride, the .
precipitation gravimeter solubility
The two most common methods for the determination of chloride ion are based on its quantitative reaction with silver ion to form silver chloride. One method involves the isolation of the silver chloride precipitate by filtration, determining its mass, and using stoichiometry to calculate the percent chloride in the sample.One fine example of gravimetric analysis which fulfill the majority of the above requirements is the determination of chloride ions in a sample. Chloride ion can be rather conveniently determined by precipitating it as silver chloride by adding silver nitrate to a sample. The precipitate is treated appropriately, filtered, dried, and weighed.For instance, in the presence of a slight excess of ammonia, silver ions form complexes with ammonia preventing the precipitation of silver chloride. Co-precipitation of other salts having low solubility in the presence of the precipitating agent will increase the quantity of precipitate.
Gravimetric Determination of Chloride Introduction The chloride content of a soluble salt, or of an aqueous solution, can be determined by precipitation of the chloride ion as silver chloride: Ag+(aq) + Cl-(aq) → AgCl(s) The silver chloride precipitate initially forms as a colloid, which is coagulated with heat. Nitric acid and a small excess .
best moisture meter for concrete floor
The chloride content of a soluble salt, or of an aqueous solution, can be determined by precipitation of the chloride ion as silver chloride: Ag+(aq) + Cl-‐(aq) → AgCl(s) The silver chloride precipitate initially forms as a colloid, which is coagulated with heat.Thermo gravimetric analysis method of analysis includes quantitative change in mass of analyte with reference to temperature. This technique is purely instrumental. . solution silver ion as precipitating agent are not a good choice as other halide ions also get precipitate along with silver chloride. 2.2 Precipitation: Among various insoluble .
best moisture meter for fiberglass boats
Atendimento humano e digital. No cartão de crédito ou débito em conta. Consulte Condições para o seu endereço! 0800 316 1515 Detalhes.
gravimetric determination of chloride as silver chloride by precipitation method|chloride anion gravimeter